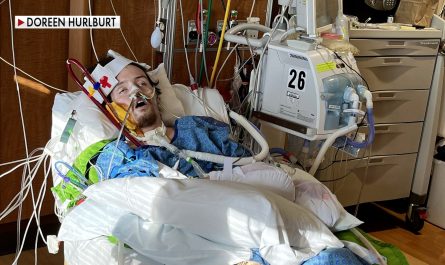
Eli Lilly’s donanemab was expected to be approved this month, but the agency has decided to convene a panel of independent experts to evaluate the drug’s safety and efficacy.Eli Lilly’s donanemab was expected to be approved this month, but the agency has decided to convene a panel of independent experts to evaluate the drug’s safety and efficacy.Read MoreDrugs (Pharmaceuticals), Alzheimer’s Disease, Regulation and Deregulation of Industry, Clinical Trials, Eli Lilly and Company, Food and Drug Administration, donanemabNYT > Health